Uk Drug Regulator
- All
- News
- Videos
-

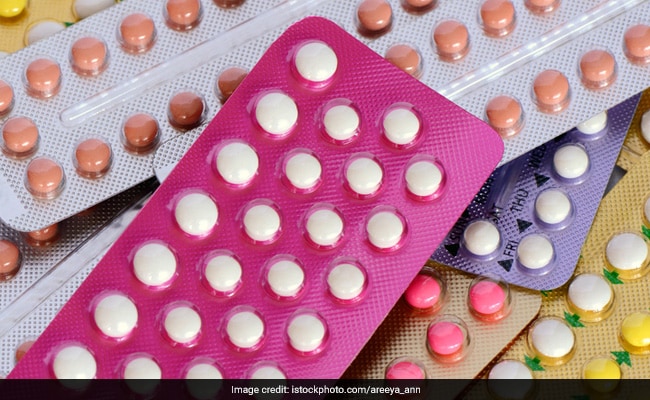
Ensure Weight Loss Drugs Are Safe, Legal Before Use In 2026: UK Health Agency
- Tuesday December 30, 2025
- Health | Indo-Asian News Service
New Year is the time for many people to pledge reforms in habits and lifestyle, something resorting to drastic measures, but UK's Medicines and Healthcare products Regulatory Agency (MHRA) has urged the people to make sure an
-
 www.ndtv.com
www.ndtv.com
-

India Drug Regulator Allows Serum Insititute To Export Its Malaria Vaccine To UK
- Thursday September 29, 2022
- India News | Press Trust of India
India's drug regulator has allowed the export of the first produced-in-India vaccine against malaria, developed by scientists at the University of Oxford and manufactured by Serum Institute to the UK.
-
 www.ndtv.com
www.ndtv.com
-

UK Approves An Updated Moderna Vaccine Which Targets Omicron Variant
- Monday August 15, 2022
- World News | Agence France-Presse
The UK's drug regulator said Monday it has approved an updated Moderna vaccine against the coronavirus that targets the Omicron variant as well as the original form.
-
 www.ndtv.com
www.ndtv.com
-

UK Approves GlaxoSmithKline Drug, Appears Effective Against Omicron
- Thursday December 2, 2021
- World News | Agence France-Presse
British regulators on Thursday approved GlaxoSmithKline drug sotrovimab to treat those at high risk of developing severe Covid-19 symptoms, with the manufacturer saying it "retains activity" against the new Omicron variant.
-
 www.ndtv.com
www.ndtv.com
-

Serum Institute Seeks India Trial Of A Second Covid Vaccine
- Friday January 29, 2021
- India News | Reuters
The Serum Institute of India, the world's biggest vaccine makers, has sought the drug regulator's permission to conduct a small domestic trial of the Novavax Inc COVID-19 vaccine that was found to be 89.3% effective in a UK trial, its CEO told Reuters on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Oxford Vaccine Likely To Be First To Get Approval In India, Say Officials
- Saturday December 26, 2020
- India News | Press Trust of India
With preparations underway for a possible vaccine-rollout by January, the Indian drug regulator is looking at the UK, which sources believe may give its nod to the Oxford COVID-19 vaccine next week, before deciding on giving emergency use authorisation to the Serum Institute that is manufacturing the shots.
-
 www.ndtv.com
www.ndtv.com
-

Oxford-AstraZeneca Covid Vaccine Submitted For UK Approval
- Wednesday December 23, 2020
- World News | Agence France-Presse
The University of Oxford and drug manufacturer AstraZeneca have applied to the UK health regulator for permission to roll out their Covid-19 vaccine, Health Minister Matt Hancock said Wednesday.
-
 www.ndtv.com
www.ndtv.com
-

UK Asks Regulator To Study AstraZeneca Vaccine
- Friday November 27, 2020
- World News | Agence France-Presse
The British government said on Friday it has asked its independent medicines regulator to assess AstraZeneca's coronavirus vaccine as part of the formal approval process for the drug to be rolled out by the end of the year.
-
 www.ndtv.com
www.ndtv.com
-

UK Suspends Hydroxychloroquine Trial For COVID-19
- Wednesday June 17, 2020
- World News | Reuters
Britain's drug regulator on Tuesday instructed scientists trialling the use of malaria drug hydroxychloroquine for the treatment or prevention of COVID-19 to suspend the recruitment of participants.
-
 www.ndtv.com
www.ndtv.com
-

Ensure Weight Loss Drugs Are Safe, Legal Before Use In 2026: UK Health Agency
- Tuesday December 30, 2025
- Health | Indo-Asian News Service
New Year is the time for many people to pledge reforms in habits and lifestyle, something resorting to drastic measures, but UK's Medicines and Healthcare products Regulatory Agency (MHRA) has urged the people to make sure an
-
 www.ndtv.com
www.ndtv.com
-

India Drug Regulator Allows Serum Insititute To Export Its Malaria Vaccine To UK
- Thursday September 29, 2022
- India News | Press Trust of India
India's drug regulator has allowed the export of the first produced-in-India vaccine against malaria, developed by scientists at the University of Oxford and manufactured by Serum Institute to the UK.
-
 www.ndtv.com
www.ndtv.com
-

UK Approves An Updated Moderna Vaccine Which Targets Omicron Variant
- Monday August 15, 2022
- World News | Agence France-Presse
The UK's drug regulator said Monday it has approved an updated Moderna vaccine against the coronavirus that targets the Omicron variant as well as the original form.
-
 www.ndtv.com
www.ndtv.com
-

UK Approves GlaxoSmithKline Drug, Appears Effective Against Omicron
- Thursday December 2, 2021
- World News | Agence France-Presse
British regulators on Thursday approved GlaxoSmithKline drug sotrovimab to treat those at high risk of developing severe Covid-19 symptoms, with the manufacturer saying it "retains activity" against the new Omicron variant.
-
 www.ndtv.com
www.ndtv.com
-

Serum Institute Seeks India Trial Of A Second Covid Vaccine
- Friday January 29, 2021
- India News | Reuters
The Serum Institute of India, the world's biggest vaccine makers, has sought the drug regulator's permission to conduct a small domestic trial of the Novavax Inc COVID-19 vaccine that was found to be 89.3% effective in a UK trial, its CEO told Reuters on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Oxford Vaccine Likely To Be First To Get Approval In India, Say Officials
- Saturday December 26, 2020
- India News | Press Trust of India
With preparations underway for a possible vaccine-rollout by January, the Indian drug regulator is looking at the UK, which sources believe may give its nod to the Oxford COVID-19 vaccine next week, before deciding on giving emergency use authorisation to the Serum Institute that is manufacturing the shots.
-
 www.ndtv.com
www.ndtv.com
-

Oxford-AstraZeneca Covid Vaccine Submitted For UK Approval
- Wednesday December 23, 2020
- World News | Agence France-Presse
The University of Oxford and drug manufacturer AstraZeneca have applied to the UK health regulator for permission to roll out their Covid-19 vaccine, Health Minister Matt Hancock said Wednesday.
-
 www.ndtv.com
www.ndtv.com
-

UK Asks Regulator To Study AstraZeneca Vaccine
- Friday November 27, 2020
- World News | Agence France-Presse
The British government said on Friday it has asked its independent medicines regulator to assess AstraZeneca's coronavirus vaccine as part of the formal approval process for the drug to be rolled out by the end of the year.
-
 www.ndtv.com
www.ndtv.com
-

UK Suspends Hydroxychloroquine Trial For COVID-19
- Wednesday June 17, 2020
- World News | Reuters
Britain's drug regulator on Tuesday instructed scientists trialling the use of malaria drug hydroxychloroquine for the treatment or prevention of COVID-19 to suspend the recruitment of participants.
-
 www.ndtv.com
www.ndtv.com