Bharat Biotech
- All
- News
- Videos
- Photos
-

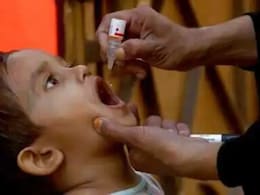
Bharat Biotech Unveils Next-Gen Oral Cholera Vaccine To Address Global Health Gaps
- Tuesday August 27, 2024
- India News | IANS
HILLCHOL (BBV131), a novel single-strain vaccine is to be administered orally on day 0 and day 14. It is suitable for babies older than one year.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Launches Oral Cholera Vaccine After Successful Phase 3 Trial
- Tuesday August 27, 2024
- India News | Press Trust of India
Bharat Biotech International Ltd on Tuesday announced the launch of Hillchol, a novel single-strain oral cholera vaccine.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Adds Top Medical Body ICMR As Co-Owner Of Covid Vaccine Patent
- Sunday June 23, 2024
- India News | Asian News International
Bharat Biotech has added the Indian Council of Medical Research (ICMR) as co-owner of the Covid-19 vaccine patent.
-
 www.ndtv.com
www.ndtv.com
-

"Remarkable Influence": Bharat Biotech Co-Founder Honoured By Johns Hopkins
- Friday May 24, 2024
- India News | Press Trust of India
Dr Krishna Ella, co-founder and Executive Chairman of Bharat Biotech, has been awarded the prestigious Dean's medal by the Johns Hopkins Bloomberg School of Public Health, the Hyderabad-based pharma company said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Top Medical Body Slams Study On Covaxin Safety, Side-Effects, Wants Apology
- Monday May 20, 2024
- India News | Reported by Parimal Kumar, Edited by Chandrashekar Srinivasan
The Indian Council of Medical Research, or ICMR, has distanced itself from a follow-up study by two Banaras Hindu University professors that said nearly a third of 926 individuals who got the India-made Covaxin shot reported serious side-effects.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Has Excellent Safety Record: Bharat Biotech On Study Citing Risks
- Thursday May 16, 2024
- India News | Indo-Asian News Service
The study, led by Banaras Hindu University (BHU), claimed that Covaxin raised the rare risk of stroke, and Guillain-Barre syndrome, among others.
-
 www.ndtv.com
www.ndtv.com
-

Over 30% Covaxin Takers Suffered Health Issues After A Year, Claims Study
- Thursday May 16, 2024
- India News | Press Trust of India
Nearly one-third of the individuals who received Bharat Biotech's anti-Covid vaccine Covaxin reported 'adverse events of special interest,' or AESI, according to a one-year follow-up study conducted at BHU.
-
 www.ndtv.com
www.ndtv.com
-

No Side Effects From Our Vaccine: Covaxin Maker Amid AstraZeneca Row
- Thursday May 2, 2024
- India News | NDTV Newsdesk
The Covaxin was developed with a single-minded focus on safety first, said its maker Bharat Biotech on Thursday, days after British pharma giant AstraZeneca admitted that its Covid vaccine can cause rare side effects.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Begins Clinical Trials Of TB Vaccine On Adults In India
- Sunday March 24, 2024
- India News | Press Trust of India
Bharat Biotech International Ltd on Sunday said it started clinical trials of the Tuberculosis vaccine Mtbvac on adults in India.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech, Sydney University Announce Pact To Advance Vaccine Research
- Tuesday November 28, 2023
- India News | Press Trust of India
Bharat Biotech and the University of Sydney Infectious Diseases Institute Tuesday announced a memorandum of understanding between them to advance vaccine research initiatives, strengthen academic-industry partnerships and
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Unveils Next-Gen Oral Cholera Vaccine To Address Global Health Gaps
- Tuesday August 27, 2024
- India News | IANS
HILLCHOL (BBV131), a novel single-strain vaccine is to be administered orally on day 0 and day 14. It is suitable for babies older than one year.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Launches Oral Cholera Vaccine After Successful Phase 3 Trial
- Tuesday August 27, 2024
- India News | Press Trust of India
Bharat Biotech International Ltd on Tuesday announced the launch of Hillchol, a novel single-strain oral cholera vaccine.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Adds Top Medical Body ICMR As Co-Owner Of Covid Vaccine Patent
- Sunday June 23, 2024
- India News | Asian News International
Bharat Biotech has added the Indian Council of Medical Research (ICMR) as co-owner of the Covid-19 vaccine patent.
-
 www.ndtv.com
www.ndtv.com
-

"Remarkable Influence": Bharat Biotech Co-Founder Honoured By Johns Hopkins
- Friday May 24, 2024
- India News | Press Trust of India
Dr Krishna Ella, co-founder and Executive Chairman of Bharat Biotech, has been awarded the prestigious Dean's medal by the Johns Hopkins Bloomberg School of Public Health, the Hyderabad-based pharma company said on Friday.
-
 www.ndtv.com
www.ndtv.com
-

Top Medical Body Slams Study On Covaxin Safety, Side-Effects, Wants Apology
- Monday May 20, 2024
- India News | Reported by Parimal Kumar, Edited by Chandrashekar Srinivasan
The Indian Council of Medical Research, or ICMR, has distanced itself from a follow-up study by two Banaras Hindu University professors that said nearly a third of 926 individuals who got the India-made Covaxin shot reported serious side-effects.
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Has Excellent Safety Record: Bharat Biotech On Study Citing Risks
- Thursday May 16, 2024
- India News | Indo-Asian News Service
The study, led by Banaras Hindu University (BHU), claimed that Covaxin raised the rare risk of stroke, and Guillain-Barre syndrome, among others.
-
 www.ndtv.com
www.ndtv.com
-

Over 30% Covaxin Takers Suffered Health Issues After A Year, Claims Study
- Thursday May 16, 2024
- India News | Press Trust of India
Nearly one-third of the individuals who received Bharat Biotech's anti-Covid vaccine Covaxin reported 'adverse events of special interest,' or AESI, according to a one-year follow-up study conducted at BHU.
-
 www.ndtv.com
www.ndtv.com
-

No Side Effects From Our Vaccine: Covaxin Maker Amid AstraZeneca Row
- Thursday May 2, 2024
- India News | NDTV Newsdesk
The Covaxin was developed with a single-minded focus on safety first, said its maker Bharat Biotech on Thursday, days after British pharma giant AstraZeneca admitted that its Covid vaccine can cause rare side effects.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech Begins Clinical Trials Of TB Vaccine On Adults In India
- Sunday March 24, 2024
- India News | Press Trust of India
Bharat Biotech International Ltd on Sunday said it started clinical trials of the Tuberculosis vaccine Mtbvac on adults in India.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech, Sydney University Announce Pact To Advance Vaccine Research
- Tuesday November 28, 2023
- India News | Press Trust of India
Bharat Biotech and the University of Sydney Infectious Diseases Institute Tuesday announced a memorandum of understanding between them to advance vaccine research initiatives, strengthen academic-industry partnerships and
-
 www.ndtv.com
www.ndtv.com