Biotech Drugs
- All
- News
- Videos
-

Could Dogs Get Their Version Of Ozempic? Scientists Test "Ozempup" For Pet Obesity
- Thursday August 21, 2025
- Lifestyle | Written by NDTV Lifestyle Desk
Biotech firm Vivani Medical has joined hands with Okava to work on a canine-friendly version of weight loss medications
-
 www.ndtv.com/lifestyle
www.ndtv.com/lifestyle
-

US Drug Regulator's Ousted Vaccine Chief Vinay Prasad Is Returning To Agency
- Tuesday August 12, 2025
- World News | Associated Press
A Food and Drug Administration official is getting his job back as the agency's top vaccine regulator, less than two weeks after he was pressured to step down at the urging of biotech executives, patient groups and conservative allies of Trump.
-
 www.ndtv.com
www.ndtv.com
-

US FDA Vaccine Chief Vinay Prasad Leaves Agency 3 Months After Appointment
- Friday August 1, 2025
- World News | Associated Press
The Food and Drug Administration's polarizing vaccine chief is leaving the agency after a brief tenure that drew the ire of biotech executives, patient groups and conservative allies of President Donald Trump.
-
 www.ndtv.com
www.ndtv.com
-

Nvidia-Backed AI Firm Unveils Drug Discovery "Breakthrough"
- Tuesday October 29, 2024
- World News | Reuters
Biotech firm Iambic Therapeutics unveiled on Tuesday what it says is a breakthrough artificial intelligence model that could drastically reduce the time and money needed to develop new drugs.
-
 www.ndtv.com
www.ndtv.com
-

After Uzbekistan Syrup Deaths, Regulator's Big Move Against Delhi Firm
- Saturday March 11, 2023
- India News | Press Trust of India
India's drug regulator directed drug manufacturers not to use propylene glycol supplied by a Delhi-based company which provided the ingredient to Marion Biotech, whose cough syrups were alleged to have led to the deaths of children in Uzbekistan.
-
 www.ndtv.com
www.ndtv.com
-

Uzbekistan Syrup Deaths: Process Initiated To Cancel Noida Firm's Licence
- Sunday March 5, 2023
- India News | Press Trust of India
The process has been initiated for cancellation of the drug licence of pharmaceutical firm Marion Biotech, which is allegedly linked with the death of 18 children in Uzbekistan who consumed their cough syrup in December 2022, officials in Noida said.
-
 www.ndtv.com
www.ndtv.com
-

Uzbekistan Child Deaths: Noida Syrup Manufacturer's Licence Suspended
- Thursday January 12, 2023
- India News | Press Trust of India
The production licence of Noida-based pharmaceutical firm Marion Biotech, allegedly linked with the deaths of children in Uzbekistan, has been suspended while the test results of its controversial cough syrup are awaited, an UP drug official said.
-
 www.ndtv.com
www.ndtv.com
-

New Nasal Vaccine Against Covid-19: Your 5-Point Guide
- Wednesday September 7, 2022
- India News | Edited by Sanjib Kumar Das
A nasal vaccine against Covid-19, iNCOVACC, developed by Bharat Biotech, has been approved by India's Central Drugs Standard Control Organisation. This is India's first intra-nasal vaccine against Covid-19
-
 www.ndtv.com
www.ndtv.com
-

Covid Nasal Vaccine's Phase 3 Trials Completed, Says Bharat Biotech Chief
- Sunday June 19, 2022
- India News | Asian News International
Dr Krishna Ella, Chairman and Managing Director of Bharat Biotech said that the clinical phase III trials of the COVID-19 nasal vaccine have been completed and the company will submit its data with Drugs Controller General of India next month.
-
 www.ndtv.com
www.ndtv.com
-

US Health Regulator Lifts Hold On Covaxin's Clinical Trials
- Tuesday May 24, 2022
- India News | Press Trust of India
The US Food and Drug Administration which has put on hold phase 2/ 3 clinical trials of Bharat Biotech's COVID-19 vaccine Covaxin, in USA, has lifted pause, according to a statement issued by Ocugen Inc, Bharat Biotech's partner for jab in US, Canada
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Cleared For 6-12 Age Group, Corbevax For 5-12-Year-Olds
- Tuesday April 26, 2022
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The Drugs Controller General of India (DCGI) today gave emergency use authorisation to Bharat Biotech's Covaxin for children between the age group of 6-12 amid an uptick in the Covid cases in schools.
-
 www.ndtv.com
www.ndtv.com
-

US Health Regulator Clears Covaxin For Clinical Trials In Adults In America
- Monday March 7, 2022
- India News | Asian News International
Bharat Biotech's COVID-19 vaccine Covaxin has been given clearance for clinical trials in adults by the US Food and Drug Administration (FDA).
-
 www.ndtv.com
www.ndtv.com
-

Novak Djokovic Holds Major Stake In Firm Developing Covid Drug: CEO
- Thursday January 20, 2022
- Agence France-Presse
Serbian tennis star Novak Djokovic, recently deported from Australia due to his coronavirus vaccine status, is co-founder and majority shareholder of a biotech firm developing a Covid treatment, the Danish company's CEO said Wednesday.
-
 sports.ndtv.com
sports.ndtv.com
-

Samsung in Talks to Buy Alzheimer’s Drug Maker Biogen for $42 Billion: Report
- Thursday December 30, 2021
- Agence France-Presse
Biogen shares surged following a report that Samsung is in talks to acquire the US biotech company for more than $40 billion (roughly Rs. 2,98,170 crore).
-
 www.gadgets360.com
www.gadgets360.com
-

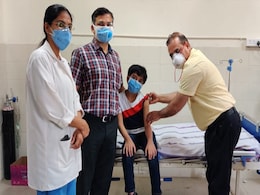
Covaxin Cleared For Children Above 12 By Drug Regulator
- Saturday December 25, 2021
- India News | Edited by Sumana Nandy
Bharat Biotech's COVID-19 vaccine Covaxin has been approved for emergency use on children between the ages of 12 and 18 by the Drugs Controller General of India (DCGI), sources said.
-
 www.ndtv.com
www.ndtv.com
-

Could Dogs Get Their Version Of Ozempic? Scientists Test "Ozempup" For Pet Obesity
- Thursday August 21, 2025
- Lifestyle | Written by NDTV Lifestyle Desk
Biotech firm Vivani Medical has joined hands with Okava to work on a canine-friendly version of weight loss medications
-
 www.ndtv.com/lifestyle
www.ndtv.com/lifestyle
-

US Drug Regulator's Ousted Vaccine Chief Vinay Prasad Is Returning To Agency
- Tuesday August 12, 2025
- World News | Associated Press
A Food and Drug Administration official is getting his job back as the agency's top vaccine regulator, less than two weeks after he was pressured to step down at the urging of biotech executives, patient groups and conservative allies of Trump.
-
 www.ndtv.com
www.ndtv.com
-

US FDA Vaccine Chief Vinay Prasad Leaves Agency 3 Months After Appointment
- Friday August 1, 2025
- World News | Associated Press
The Food and Drug Administration's polarizing vaccine chief is leaving the agency after a brief tenure that drew the ire of biotech executives, patient groups and conservative allies of President Donald Trump.
-
 www.ndtv.com
www.ndtv.com
-

Nvidia-Backed AI Firm Unveils Drug Discovery "Breakthrough"
- Tuesday October 29, 2024
- World News | Reuters
Biotech firm Iambic Therapeutics unveiled on Tuesday what it says is a breakthrough artificial intelligence model that could drastically reduce the time and money needed to develop new drugs.
-
 www.ndtv.com
www.ndtv.com
-

After Uzbekistan Syrup Deaths, Regulator's Big Move Against Delhi Firm
- Saturday March 11, 2023
- India News | Press Trust of India
India's drug regulator directed drug manufacturers not to use propylene glycol supplied by a Delhi-based company which provided the ingredient to Marion Biotech, whose cough syrups were alleged to have led to the deaths of children in Uzbekistan.
-
 www.ndtv.com
www.ndtv.com
-

Uzbekistan Syrup Deaths: Process Initiated To Cancel Noida Firm's Licence
- Sunday March 5, 2023
- India News | Press Trust of India
The process has been initiated for cancellation of the drug licence of pharmaceutical firm Marion Biotech, which is allegedly linked with the death of 18 children in Uzbekistan who consumed their cough syrup in December 2022, officials in Noida said.
-
 www.ndtv.com
www.ndtv.com
-

Uzbekistan Child Deaths: Noida Syrup Manufacturer's Licence Suspended
- Thursday January 12, 2023
- India News | Press Trust of India
The production licence of Noida-based pharmaceutical firm Marion Biotech, allegedly linked with the deaths of children in Uzbekistan, has been suspended while the test results of its controversial cough syrup are awaited, an UP drug official said.
-
 www.ndtv.com
www.ndtv.com
-

New Nasal Vaccine Against Covid-19: Your 5-Point Guide
- Wednesday September 7, 2022
- India News | Edited by Sanjib Kumar Das
A nasal vaccine against Covid-19, iNCOVACC, developed by Bharat Biotech, has been approved by India's Central Drugs Standard Control Organisation. This is India's first intra-nasal vaccine against Covid-19
-
 www.ndtv.com
www.ndtv.com
-

Covid Nasal Vaccine's Phase 3 Trials Completed, Says Bharat Biotech Chief
- Sunday June 19, 2022
- India News | Asian News International
Dr Krishna Ella, Chairman and Managing Director of Bharat Biotech said that the clinical phase III trials of the COVID-19 nasal vaccine have been completed and the company will submit its data with Drugs Controller General of India next month.
-
 www.ndtv.com
www.ndtv.com
-

US Health Regulator Lifts Hold On Covaxin's Clinical Trials
- Tuesday May 24, 2022
- India News | Press Trust of India
The US Food and Drug Administration which has put on hold phase 2/ 3 clinical trials of Bharat Biotech's COVID-19 vaccine Covaxin, in USA, has lifted pause, according to a statement issued by Ocugen Inc, Bharat Biotech's partner for jab in US, Canada
-
 www.ndtv.com
www.ndtv.com
-

Covaxin Cleared For 6-12 Age Group, Corbevax For 5-12-Year-Olds
- Tuesday April 26, 2022
- India News | Reported by Parimal Kumar, Edited by Aditi Gautam
The Drugs Controller General of India (DCGI) today gave emergency use authorisation to Bharat Biotech's Covaxin for children between the age group of 6-12 amid an uptick in the Covid cases in schools.
-
 www.ndtv.com
www.ndtv.com
-

US Health Regulator Clears Covaxin For Clinical Trials In Adults In America
- Monday March 7, 2022
- India News | Asian News International
Bharat Biotech's COVID-19 vaccine Covaxin has been given clearance for clinical trials in adults by the US Food and Drug Administration (FDA).
-
 www.ndtv.com
www.ndtv.com
-

Novak Djokovic Holds Major Stake In Firm Developing Covid Drug: CEO
- Thursday January 20, 2022
- Agence France-Presse
Serbian tennis star Novak Djokovic, recently deported from Australia due to his coronavirus vaccine status, is co-founder and majority shareholder of a biotech firm developing a Covid treatment, the Danish company's CEO said Wednesday.
-
 sports.ndtv.com
sports.ndtv.com
-

Samsung in Talks to Buy Alzheimer’s Drug Maker Biogen for $42 Billion: Report
- Thursday December 30, 2021
- Agence France-Presse
Biogen shares surged following a report that Samsung is in talks to acquire the US biotech company for more than $40 billion (roughly Rs. 2,98,170 crore).
-
 www.gadgets360.com
www.gadgets360.com
-

Covaxin Cleared For Children Above 12 By Drug Regulator
- Saturday December 25, 2021
- India News | Edited by Sumana Nandy
Bharat Biotech's COVID-19 vaccine Covaxin has been approved for emergency use on children between the ages of 12 and 18 by the Drugs Controller General of India (DCGI), sources said.
-
 www.ndtv.com
www.ndtv.com