Covid Human Trial
- All
- News
- Videos
-

Indonesia Starts Testing Homegrown Covid Vaccine On Humans
- Wednesday February 9, 2022
- World News | Agence France-Presse
Indonesia began testing a homegrown Covid-19 vaccine on humans Wednesday after getting the green light from the drug regulator as the country faces a rising wave of virus cases.
-
 www.ndtv.com
www.ndtv.com
-

World's First Trial With Voluntary Covid Exposure Found Safe In Young Adults: Study
- Wednesday February 2, 2022
- World News | Reuters
The world's first human challenge trial in which volunteers were deliberately exposed to COVID-19 to advance research into the disease was found to be safe in healthy young adults, one of the companies running the study said on Wednesday.
-
 www.ndtv.com
www.ndtv.com
-

Biological E Gets Nod For Further Trials Of Its Covid Vaccine On Children
- Friday September 3, 2021
- India News | Press Trust of India
Biological E has received approval for conducting phase II/III human clinical trial of COVID-19 vaccine candidate Corbevax on children above five years and adolescents, the Department of Biotechnology said today.
-
 www.ndtv.com
www.ndtv.com
-

4 Covid Vaccine Candidates In Human Trial Stage, Centre Tells Rajya Sabha
- Tuesday July 20, 2021
- India News | Press Trust of India
Four COVID-19 vaccine candidates are at different stages of human trials while one, developed by Genique Life Sciences, is in the advanced pre-clinical stage, Union Science and Technology Minister Jitendra Singh said on Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

US COVID-19 Vaccine Hopeful Of Using Gates Foundation Cash To Prepare For Human Trial
- Friday January 29, 2021
- World News | Reuters
Scientists who normally focus on fixing defective genes said on Friday that up to $2.1 million from the Bill & Melinda Gates Foundation will help them move their COVID-19 vaccine candidate toward 2021 human trials.
-
 www.ndtv.com
www.ndtv.com
-

India's First Homemade mRNA Vaccine Gets Permission To Start Human Trials
- Friday December 11, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Vaibhav Tiwari
An anti-Coronavirus vaccine being developed by the Pune-based company, Gennova, has become the first indigenous mRNA candidate to have received approval to initiate human clinical trial, a central government statement has said.
-
 www.ndtv.com
www.ndtv.com
-

Gennova Biopharmaceuticals Gets Conditional Nod For Human Trial Of Its Covid Vaccine
- Thursday December 10, 2020
- India News | Press Trust of India
The Drugs Controller General of India (DCGI) on Wednesday granted conditional permission for phases 1 and 2 human clinical trial of the COVID-19 vaccine candidate developed by the Pune-based Gennova Biopharmaceuticals Ltd in collaboration with HDT, USA, officials said.
-
 www.ndtv.com
www.ndtv.com
-

UAE Says China-Developed Sinopharm Covid Vaccine Has 86% Efficacy
- Wednesday December 9, 2020
- World News | Reuters
An experimental coronavirus vaccine developed by China's Sinopharm has 86% efficacy against the virus, the United Arab Emirates health ministry said on Wednesday, citing an interim analysis of a human trial underway there.
-
 www.ndtv.com
www.ndtv.com
-

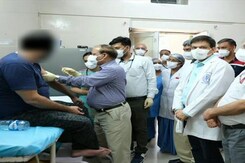
Bharat Biotech's COVID-19 Vaccine 'Covaxin' Commences Phase-3 Trial At AIIMS: Report
- Thursday November 26, 2020
- India News | Press Trust of India
Phase-three of human clinical trial of the India-made COVID-19 vaccine candidate Covaxin began at AIIMS in Delhi on Thursday with Dr MV Padma Srivastava, the chief of Neurosciences Centre at the premier institute, and three other volunteers receiving the first dose.
-
 www.ndtv.com
www.ndtv.com
-

Hyderabad-Based Firm Biological E Starts Human Trials Of Covid Vaccine Candidate
- Monday November 16, 2020
- India News | Reuters
Biological E. Ltd has started human trials of its COVID-19 vaccine candidate and expects results by February, the drugmaker said on Monday.
-
 www.ndtv.com
www.ndtv.com
-

Indonesia Starts Testing Homegrown Covid Vaccine On Humans
- Wednesday February 9, 2022
- World News | Agence France-Presse
Indonesia began testing a homegrown Covid-19 vaccine on humans Wednesday after getting the green light from the drug regulator as the country faces a rising wave of virus cases.
-
 www.ndtv.com
www.ndtv.com
-

World's First Trial With Voluntary Covid Exposure Found Safe In Young Adults: Study
- Wednesday February 2, 2022
- World News | Reuters
The world's first human challenge trial in which volunteers were deliberately exposed to COVID-19 to advance research into the disease was found to be safe in healthy young adults, one of the companies running the study said on Wednesday.
-
 www.ndtv.com
www.ndtv.com
-

Biological E Gets Nod For Further Trials Of Its Covid Vaccine On Children
- Friday September 3, 2021
- India News | Press Trust of India
Biological E has received approval for conducting phase II/III human clinical trial of COVID-19 vaccine candidate Corbevax on children above five years and adolescents, the Department of Biotechnology said today.
-
 www.ndtv.com
www.ndtv.com
-

4 Covid Vaccine Candidates In Human Trial Stage, Centre Tells Rajya Sabha
- Tuesday July 20, 2021
- India News | Press Trust of India
Four COVID-19 vaccine candidates are at different stages of human trials while one, developed by Genique Life Sciences, is in the advanced pre-clinical stage, Union Science and Technology Minister Jitendra Singh said on Tuesday.
-
 www.ndtv.com
www.ndtv.com
-

US COVID-19 Vaccine Hopeful Of Using Gates Foundation Cash To Prepare For Human Trial
- Friday January 29, 2021
- World News | Reuters
Scientists who normally focus on fixing defective genes said on Friday that up to $2.1 million from the Bill & Melinda Gates Foundation will help them move their COVID-19 vaccine candidate toward 2021 human trials.
-
 www.ndtv.com
www.ndtv.com
-

India's First Homemade mRNA Vaccine Gets Permission To Start Human Trials
- Friday December 11, 2020
- India News | Reported by Sukirti Dwivedi, Edited by Vaibhav Tiwari
An anti-Coronavirus vaccine being developed by the Pune-based company, Gennova, has become the first indigenous mRNA candidate to have received approval to initiate human clinical trial, a central government statement has said.
-
 www.ndtv.com
www.ndtv.com
-

Gennova Biopharmaceuticals Gets Conditional Nod For Human Trial Of Its Covid Vaccine
- Thursday December 10, 2020
- India News | Press Trust of India
The Drugs Controller General of India (DCGI) on Wednesday granted conditional permission for phases 1 and 2 human clinical trial of the COVID-19 vaccine candidate developed by the Pune-based Gennova Biopharmaceuticals Ltd in collaboration with HDT, USA, officials said.
-
 www.ndtv.com
www.ndtv.com
-

UAE Says China-Developed Sinopharm Covid Vaccine Has 86% Efficacy
- Wednesday December 9, 2020
- World News | Reuters
An experimental coronavirus vaccine developed by China's Sinopharm has 86% efficacy against the virus, the United Arab Emirates health ministry said on Wednesday, citing an interim analysis of a human trial underway there.
-
 www.ndtv.com
www.ndtv.com
-

Bharat Biotech's COVID-19 Vaccine 'Covaxin' Commences Phase-3 Trial At AIIMS: Report
- Thursday November 26, 2020
- India News | Press Trust of India
Phase-three of human clinical trial of the India-made COVID-19 vaccine candidate Covaxin began at AIIMS in Delhi on Thursday with Dr MV Padma Srivastava, the chief of Neurosciences Centre at the premier institute, and three other volunteers receiving the first dose.
-
 www.ndtv.com
www.ndtv.com
-

Hyderabad-Based Firm Biological E Starts Human Trials Of Covid Vaccine Candidate
- Monday November 16, 2020
- India News | Reuters
Biological E. Ltd has started human trials of its COVID-19 vaccine candidate and expects results by February, the drugmaker said on Monday.
-
 www.ndtv.com
www.ndtv.com